Paxlovid significantly reduces the risk of hospitalizations and death from COVID-19 in older individuals, but not for younger people, something payers and providers might want to keep in mind as the medication will stop being offered for free in the U.S. when the public health emergency ends in May.
Researchers with Public Health Ontario stated in their study in the Canadian Medical Association Journal that “we observed substantial variation in the absolute risk reduction, which suggests that use of nirmatrelvir–ritonavir [Paxlovid] in populations at lower risk of COVID-19 may have limited population health benefits with important implications for its cost-effectiveness evaluations.”
The researchers examined data on adults with mild disease who tested positive in a polymerase chain reaction (PCR) test for COVID-19 between April 4 and Aug. 31, 2022. They compared 8,876 individuals treated with Paxlovid with 168,699 who did not get the drug. The data were collected as the omicron variant emerged.
The study’s lead author, Kevin Schwartz, M.D., said in a press release: “Our study, in conjunction with previous clinical trials and observational research, supports the effectiveness of nirmatrelvir–ritonavir at reducing hospital admission from COVID-19 and all-cause death.”
Experts consider Paxlovid the most effective treatment against COVID-19, but providers in the U.S. seem hesitant to prescribe it even for vulnerable populations. A poll taken last July by the COVID States Project, a consortium of universities, found that only about 20% of adults 65 or older who’ve tested positive for COVID-19 since January 2022 had been prescribed the medication.
There are also concerns that Paxlovid might not interact well with other medications.
Public Health Ontario researchers found “based on the data sources used in this study, we were unable to confirm whether patients were actually taking interacting medications concurrently or determine if any potential drug–drug interactions were appropriately mitigated at the time of nirmatrelvir–ritonavir prescribing, which may have affected our estimation of drug–drug interactions. Our results suggest that patients with COVID-19 and level 2 drug–drug interactions can be effectively treated with nirmatrelvir–ritonavir, and that prescribers and pharmacists are key in the evaluation for and mitigation of drug–drug interactions.”
Another concern about Paxlovid is that individuals might experience a rebound effect—a reemergence of COVID-19 infection after taking the prescribed course of Paxlovid. Famous “rebounders” include President Joe Biden, Rochelle Walensky, M.D., the director of the Centers for Disease Control and Prevention (CDC), and Anthony Fauci, M.D., former top White House medical adviser.
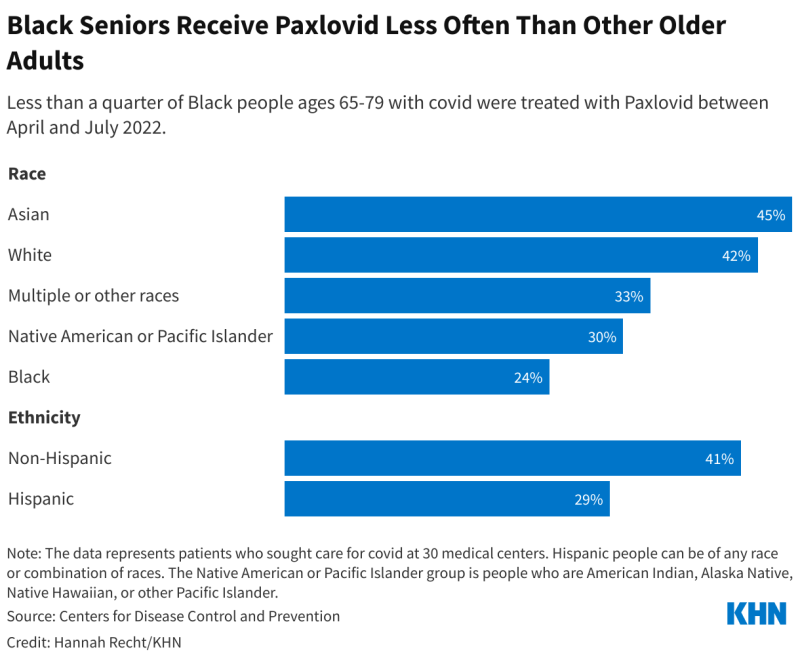
In addition, CDC researchers found in a study published last October that Black and Hispanic individuals were less likely to have access to Paxlovid than white individuals. In its Morbidity and Mortality Weekly Report, the CDC noted that “during April–July 2022, the percentage of COVID-19 patients aged ≥20 years treated with Paxlovid was 36% and 30% lower among Black and Hispanic patients than among White and non-Hispanic patients, respectively. These disparities existed among all age groups and patients with immunocompromise.”
Public Health Ontario researchers found that Paxlovid prevented one case of serious COVID-19 for every 62 people treated with the medication. In terms of evaluating cost-effectiveness, the study states that “differences between strata had overlapping [confidence intervals]; we did not compare these statistically, and any observed differences should be interpreted with caution. Further stratification by different age groups and risk factors may be helpful in the future to evaluate the benefit in those younger than 70 years of age.”
Schwartz said the study “highlights the importance of testing for SARS-CoV-2 if you have symptoms, and access to Paxlovid for those at risk for severe COVID-19. If you test positive for COVID-19, are over 60 years of age, or if you have other risk factors for severe infection, such as chronic medical conditions or are undervaccinated, contact your healthcare provider or pharmacy within five days of symptoms starting and ask about Paxlovid.”